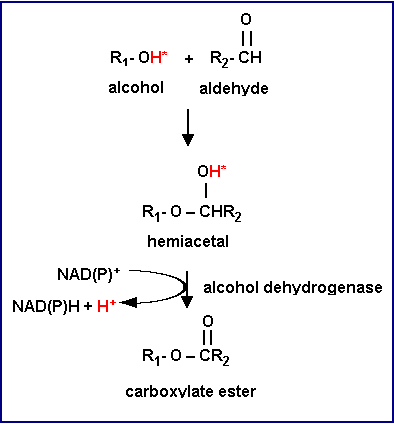
Carboxylate esters are used as flavoring ingredients in food industry, and as chemical and pharmaceutical intermediates in chemical processes. For example, isoamyl acetate and ethyl acetate are known to affect flavor quality during the beer fermentation. Butylacetate has a strong pineapple flavor. Chemical synthesis of carboxylate esters has suffered from a low stereo-selectivity of esters and environmental concerns of catalytic processes. The biological production of carboxylate esters is being developed using metabolic engineering and enzymatic technologies. Yeast, fruit and plants produce flavors naturally. In the final step, however, three enzymes can be involved in ester bond formation. One, lipase or esterase can catalyze the dehydration reaction between alcohols and carboxylic acids. Two, alcohol acyltransferase converts alcohols and acyl-CoAs to their corresponding esters. Three, typical alcohol dehydrogenases react in the reduction of an aldehyde or the oxidation of an alcohol. Some enzymes can oxidize hemiacetal compounds that are favorably formed in alcohol and aldehyde mixtures to yield carboxylate esters. This reaction requires NAD(P)+ as a hydrogen acceptor. The mechanism of carboxylate ester production by alcohol dehydrogenase is shown in the Figure. |